
1,1,1,2-Tetrafluoroethane
|
|
|||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1,2-Tetrafluoroethane
|
|||
| Other names
Freon 134a
Dymel 134a Forane 134a Genetron 134a HFA-134a HFC-134a R-134a Suva 134a Norflurane |
|||
| Identifiers | |||
|
CAS Number
|
|
||
|
3D model (JSmol)
|
|
||
| ChemSpider |
|
||
| ECHA InfoCard | 100.011.252[51] | ||
| EC Number | 212-377-0 | ||
| KEGG |
|
||
| RTECS number | KI8842500 | ||
| UNII |
|
||
|
CompTox Dashboard(EPA)
|
|
||
|
InChI
|
|||
|
SMILES
|
|||
| Properties | |||
| CH2FCF3[1] | |||
| Molar mass | 102.03 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 0.00425 g/cm3, gas | ||
| Melting point | −103.3 °C (−153.9 °F; 169.8 K) | ||
| Boiling point | −26.3 °C (−15.3 °F; 246.8 K) | ||
|
Solubility in water
|
0.15 wt% | ||
| Hazards | |||
| Main hazards | Asphyxiant | ||
| Safety data sheet | See: data page | ||
| GHS pictograms |  |
||
| GHS signal word | WARNING | ||
|
GHS hazard statements
|
H280 | ||
|
GHS precautionary statements
|
P410+403 | ||
| NFPA 704 |
 0
1
1
|
||
| Flash point | 250 °C (482 °F; 523 K) | ||
| Related compounds | |||
|
Related refrigerants
|
Difluoromethane Pentafluoroethane |
||
|
Related compounds
|
2-Chloro- 1,1,1,2-tetrafluoroethane 1,1,1-Trichloroethane |
||
| Supplementary data page | |||
|
Structure and properties
|
Refractive index(n), Dielectric constant (εr), etc. |
||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
|
Spectral data
|
UV, IR, NMR, MS | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
1,1,1,2-Tetrafluoroethane(also known asnorflurane(INN),R-134a,Freon 134a,Forane 134a,Genetron 134a,Florasol 134a,Suva 134a, orHFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties similar to R-12 (dichlorodifluoromethane) but with insignificant ozone depletion potential and a significantly lower global warming potential (1,430, compared to R-12’s GWP of 10,900).[2]It has the formula CH2FCF3 and aboiling pointof −26.3 °C (−15.34 °F) at atmospheric pressure. R-134a cylinders are colored light blue.[3]Attempts at phasing out its use as a refrigerant with substances that have lower global warming potential, such as HFO-1234yf, are underway.
|
|
|||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1,2-Tetrafluoroethane
|
|||
| Other names
Freon 134a
Dymel 134a Forane 134a Genetron 134a HFA-134a HFC-134a R-134a Suva 134a Norflurane |
|||
| Identifiers | |||
|
CAS Number
|
|
||
|
3D model (JSmol)
|
|
||
| ChemSpider |
|
||
| ECHA InfoCard | 100.011.252[51] | ||
| EC Number | 212-377-0 | ||
| KEGG |
|
||
| RTECS number | KI8842500 | ||
| UNII |
|
||
|
CompTox Dashboard(EPA)
|
|
||
|
InChI
|
|||
|
SMILES
|
|||
| Properties | |||
| CH2FCF3[1] | |||
| Molar mass | 102.03 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 0.00425 g/cm3, gas | ||
| Melting point | −103.3 °C (−153.9 °F; 169.8 K) | ||
| Boiling point | −26.3 °C (−15.3 °F; 246.8 K) | ||
|
Solubility in water
|
0.15 wt% | ||
| Hazards | |||
| Main hazards | Asphyxiant | ||
| Safety data sheet | See: data page | ||
| GHS pictograms |  |
||
| GHS signal word | WARNING | ||
|
GHS hazard statements
|
H280 | ||
|
GHS precautionary statements
|
P410+403 | ||
| NFPA 704 |
 0
1
1
|
||
| Flash point | 250 °C (482 °F; 523 K) | ||
| Related compounds | |||
|
Related refrigerants
|
Difluoromethane Pentafluoroethane |
||
|
Related compounds
|
2-Chloro- 1,1,1,2-tetrafluoroethane 1,1,1-Trichloroethane |
||
| Supplementary data page | |||
|
Structure and properties
|
Refractive index(n), Dielectric constant (εr), etc. |
||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
|
Spectral data
|
UV, IR, NMR, MS | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Uses
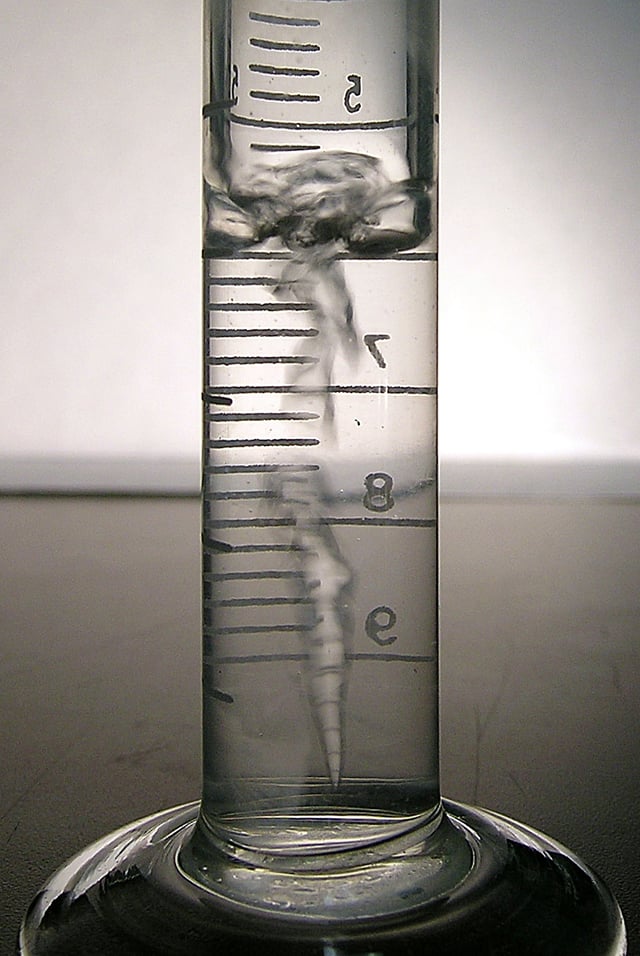
Tetrafluoroethane, when compressed as inside gas duster cans, is a clear liquid which boils when exposed to atmospheric pressure at room temperature (as seen here) and can be extracted from common “canned air” canisters by simply inverting them during use.
1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a “high-temperature” refrigerant for domestic refrigeration and automobile air conditioners. These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12 and retrofit kits are available to convert units that were originally R-12-equipped. Other uses include plastic foam blowing, as a cleaning solvent, a propellant for the delivery of pharmaceuticals (e.g. bronchodilators), wine cork removers, gas dusters, such as Dust-Off, and in air driers for removing the moisture from compressed air. 1,1,1,2-Tetrafluoroethane has also been used to cool computers in some overclocking attempts. It is the refrigerant used in plumbing pipe freeze kits. It is also commonly used as a propellant for airsoft airguns. The gas is often mixed with a silicone-based lubricant.
1,1,1,2-Tetrafluoroethane is also being considered as an organic solvent suitable for extraction of flavor and fragrance compounds, as a possible alternative to other organic solvents and supercritical carbon dioxide.[4][5]It can also be used as a solvent in organic chemistry, both in liquid andsupercritical fluid.[6]It is used in the resistive plate chamber particle detectors in theLarge Hadron Collider.[7][8]It is also used for other types of particle detectors, e.g. some cryogenic particle detectors.[9]It can be used as an alternative to sulfur hexafluoride inmagnesiumsmelting as a shielding gas.[10]
1,1,1,2-Tetrafluoroethane is also being considered as an alternative to sulfur hexafluoride as a dielectric gas.[11]Its arc-quenching properties are poor, but its dielectric properties are fairly good.
Climate change considerations
Recently, 1,1,1,2-tetrafluoroethane has been subject to use restrictions due to its contribution toclimate change.[12]It has a global warming potential of 1300.[13]
The Society of Automotive Engineers (SAE) has proposed 1,1,1,2-tetrafluoroethane to be best replaced by a new fluorochemical refrigerant HFO-1234yf (CF3CF=CH2) in automobile air-conditioning systems.[14]California may also prohibit the sale of canned 1,1,1,2-tetrafluoroethane to individuals to avoid non-professional recharge of air conditioners.[15]A ban had been in place in Wisconsin since October 1994 under ATCP 136 prohibiting sales of container sizes holding less than 15 lbs of 1,1,1,2-tetrafluoroethane, but this restriction applied only when the chemical was intended to be a refrigerant. However, the ban was lifted in Wisconsin in 2012.[16]During the time that it was active, this Wisconsin-specific ban contained loopholes. For example, it was legal for a person to purchase gas duster containers with any amount of the chemical because in that instance the chemical is neither intended to be a refrigerant [16]nor is HFC-134a included in the § 7671a listing of class I and class II substances.[17]
History
1,1,1,2-Tetrafluoroethane first appeared in the early 1990s as a replacement for dichlorodifluoromethane (R-12), which has ozone depleting properties.[18]1,1,1,2-Tetrafluoroethane has been atmospherically modeled for its impact on depleting ozone and as a contributor to global warming. Research suggests that over the past 10 years the concentration of 1,1,1,2-tetrafluoroethane has increased significantly in the Earth’s atmosphere, with a recent study revealing a doubling in atmospheric concentration between 2001 and 2004.[19]It has insignificant ozone depletion potential (ozone layer), significantglobal warmingpotential (100-yr GWP = 1430)[20]and negligible acidification potential (acid rain). Because of its high GWP, 1,1,1,2-tetrafluoroethane has been banned from use in the European Union, starting with cars in 2011 and phasing out completely by 2017.[21]
Production
Tetrafluoroethane is typically made by reacting trichloroethylene with hydrogen fluoride:[22]
- CHCl=CCl2
- 4 HF → CH
- 3 HCl
Safety
Mixtures with air of the gas 1,1,1,2-tetrafluoroethane are not flammable at atmospheric pressure and temperatures up to 100 °C (212 °F). However, mixtures with high concentrations of air at elevated pressure and/or temperature can beignited.[23]Contact of 1,1,1,2-tetrafluoroethane with flames or hot surfaces in excess of 250 °C (482 °F) may cause vapor decomposition and the emission of toxic gases including hydrogen fluoride and carbonyl fluoride.[24]1,1,1,2-Tetrafluoroethane itself has an LD50 of 1,500 g/m3 in rats, making it relatively non-toxic, apart from the dangers inherent to inhalant abuse. Its gaseous form is denser than air and will displace air in the lungs. This can result inasphyxiationif excessively inhaled.[25][26]This is what contributes to most deaths by inhalant abuse.
Aerosol cans containing 1,1,1,2-tetrafluoroethane, when inverted, become effective freeze sprays. Under pressure, 1,1,1,2-tetrafluoroethane is compressed into a liquid, which upon vaporization absorbs a significant amount of thermal energy. As a result, it will greatly lower the temperature of any object it contacts as it evaporates. This can result in frostbite when it contacts skin, as well as blindness upon eye contact.
Medical use
For its medical uses, 1,1,1,2-tetrafluoroethane has the generic name norflurane. It is used as propellant for some metered dose inhalers.[27]It is considered safe for this use.[28][29][30]In combination with pentafluoropropane, it is used as a topical vapocoolant spray for numbing boils before curettage.[31][32]It has also been studied as a potential inhalational anesthetic,[33]but it is nonanaesthetic at doses used in inhalers.[28]
See also
-
List of refrigerants
-
Dichlorodifluoromethane
-
1,1,1,2-Tetrafluoroethane (data page)
-
Tetrabromoethane
-
Tetrachloroethane



